Resource • Case Study
Strict Processes Rescue Complex MS Trial

Biorasi’s Project Management-first Approach
Allows Rapid Feasibility and Site Startup to Meet Critical Deadlines
The Challenge:
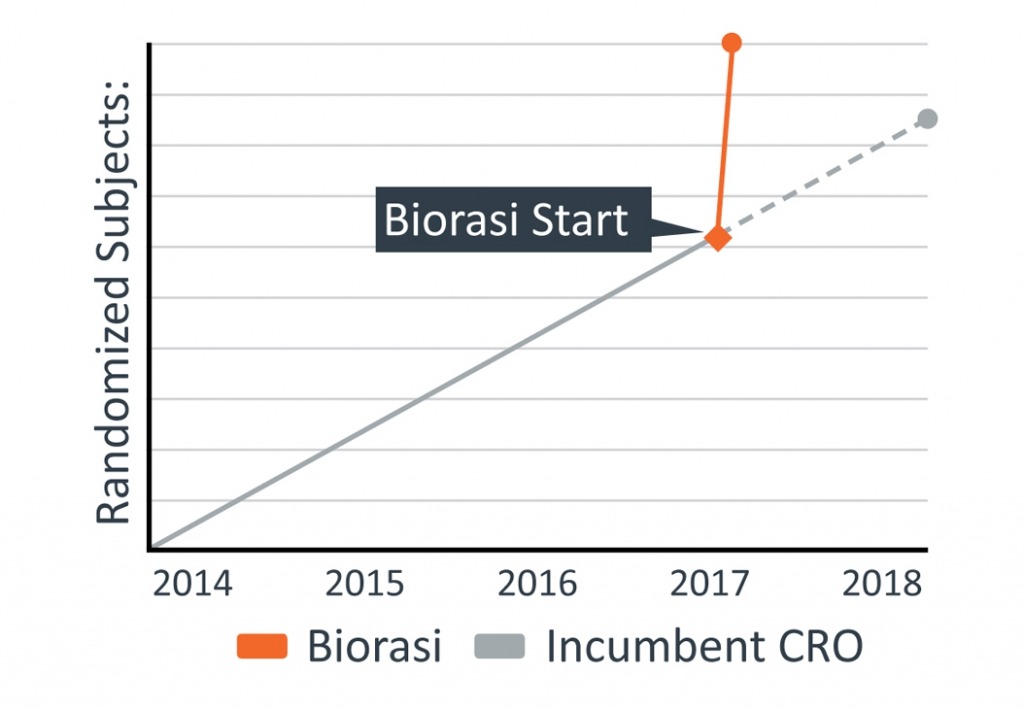
A specialty pharmaceutical company engaged Biorasi to rescue their phase 2 clinical trial for treatment of spasticity caused by multiple sclerosis (MS). The incumbent CRO was not meeting enrollment expectations and a further delay by the contracted distribution depot was causing the study to miss key milestones that would prevent timely submission of the clinical study report (CSR). At the current rate, delivery of the CSR would be completed one year later than original projections. Biorasi was tasked with the aggressive goal of enrolling twenty patients in two months using a limited number of sites. Clinical trials in MS are already challenging due to an incomplete understanding of the pathophysiology of the disease and finding patients to participate in MS trials can be tricky, especially with such a tight time constraint. What was needed was an innovative solution that could be implemented immediately, and executed efficiently without putting quality at risk.
The Solution:
Biorasi kicked off the rescue project immediately and the project management team devised a strategy to complete the CSR by the sponsor’s requested deadline. Biorasi’s multi-venue neurology site database was leveraged and predictive analytics identified top performing, high quality sites with known ability to get up and running efficiently as well as consistently enroll MS patients at high rates. Additional feasibility was performed in the first week including expedited site visits to facilitate site selection. Aggressive pre-screening activities were implemented and monitored in real time to increase the number of quality candidates available to the sites. Finally, to further mitigate the time crunch, on-going data cleaning was performed so that database lock could be initiated three weeks prior to site closeout and time could be saved on statistical analysis.
The Result:
The project kicked off on the target date. Thanks to our team’s creative execution strategy, nine sites were added and qualified patients were already available as soon as the sites were initiated. The sponsor’s ambitious enrollment goals were surpassed; 35 patients were screened and 34 were randomized in only 17 days, over a month before the sponsor requested. Database lock was achieved prior to the proposed deadline allowing for timely statistical analyses and submission of the final CSR. The project was successfully rescued even faster than proposed to ensure achievement of the sponsor’s goals.