Resource • Case Study
Dermatology Expertise Helps Biorasi Recruit In Challenging Conditions

Intelligent Processes Overcome Enrollment Barriers
The Challenge:
Patient compliance was a serious concern, particularly regarding the reduction of sun exposure. Running a dermatology trial, especially in the middle of summer, can greatly affect patient compliance and the resulting, potential flare-ups in any dermatological condition.
The Solution:
Biorasi implemented various layers of risk mitigation to assist in offsetting any foreseen compliance issues. To counteract patient non-compliance during summer months, summer enrollment was planned to incorporate a higher percentage of northern states where temperature severity is not as commonly noted. Furthermore, Biorasi’s feasibility process was two-fold incorporating both our project management and clinical teams from the get-go to ensure site enrollment estimates were as accurate as possible to realistically forecast expected enrollment and discontinuation numbers. Extensive site and subject education was incorporated into the study to ensure subjects were actively engaged in complying with study requirements and to ensure sites were equally well-trained on effective means of subject communication throughout the study.
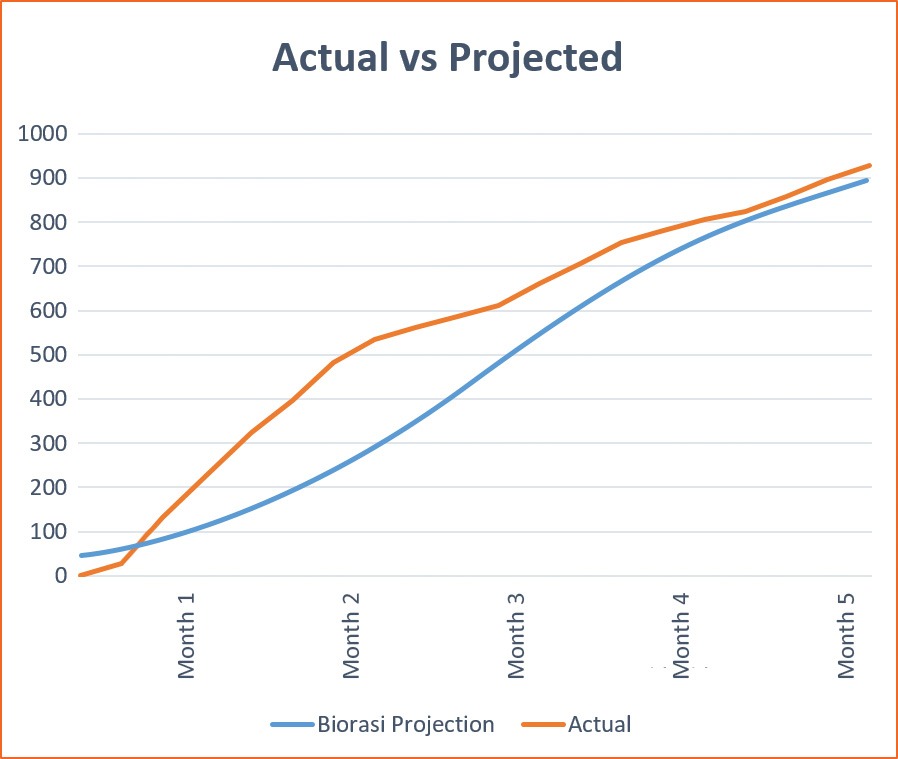
The Result:
The study was able to surpass initial enrollment projections, enrolling 963 subjects in under 5 months. With the incorporation of 5 study monitors to effectively train and track site progress and subject compliance, sites were able to successfully meet their enrollment goals and avoid unnecessary subject discontinuations due to treatment failures and subject non-compliance.